CFR21 Software
CFR21 Software

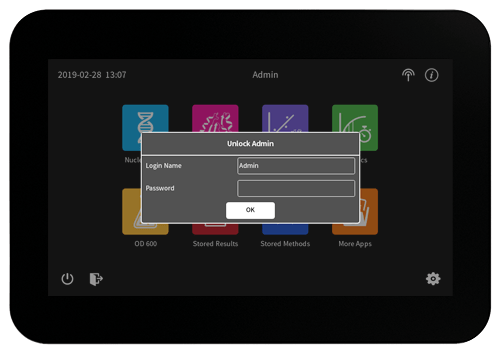
User Management
Individual Role Based Access Control (RBAC) provides password protected access and control of the NanoPhotometer®. Create multiple user accounts with different access rights which are handled in a hierarchic structure. User role options are Administrator, Reviewer, Power User, and User. Organize users into working groups to facilitate access of shared data and stored methods within a lab. Option for increased transparency with Four Eye Administrator Rights. Various password settings are available within the CFR21 software – for example secure password and password expiration options. Effectively improve data security and fulfill audit regulations easily with flexible and appropriate RBAC user management solutions. All features can be enabled or disabled on demand to meet your laboratory needs.
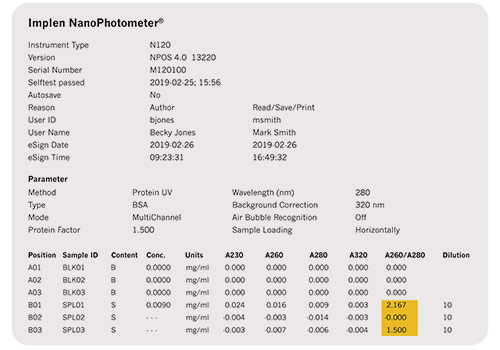
Electronic Signature
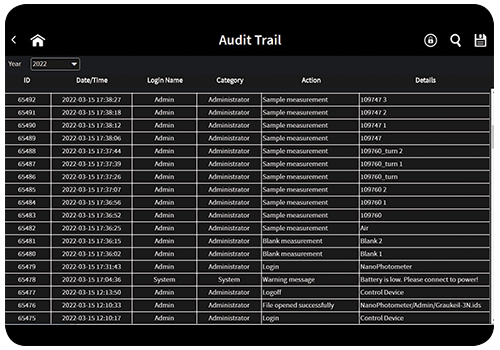
Audit Trail
Maintenance

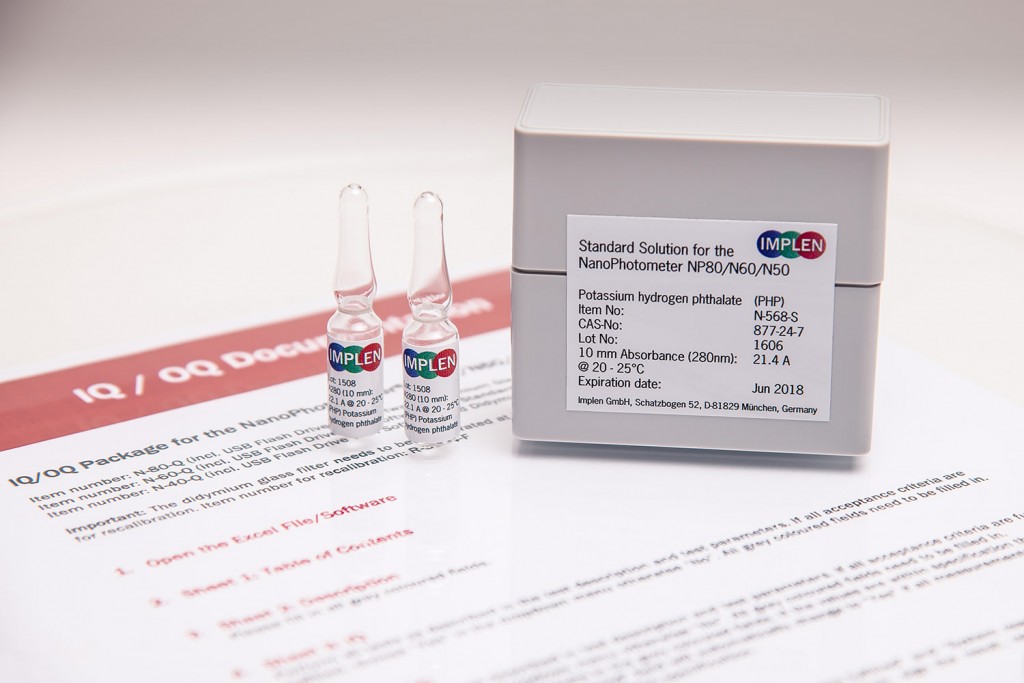
IQ/OQ Package
LIMS Integration
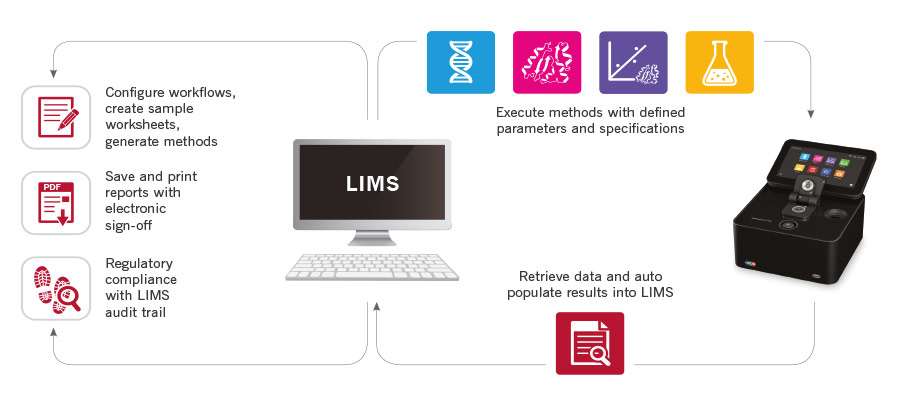 Add more efficiency to your workflow by integrating the NanoPhotometer®
with your LIMS to control processes, eliminate errors and save time.
The NanoPhotometer® can be integrated with any LIMS software provider.
Add more efficiency to your workflow by integrating the NanoPhotometer®
with your LIMS to control processes, eliminate errors and save time.
The NanoPhotometer® can be integrated with any LIMS software provider.