Instrument Interface
Instrument Interface
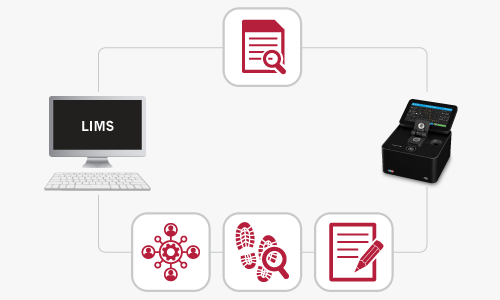
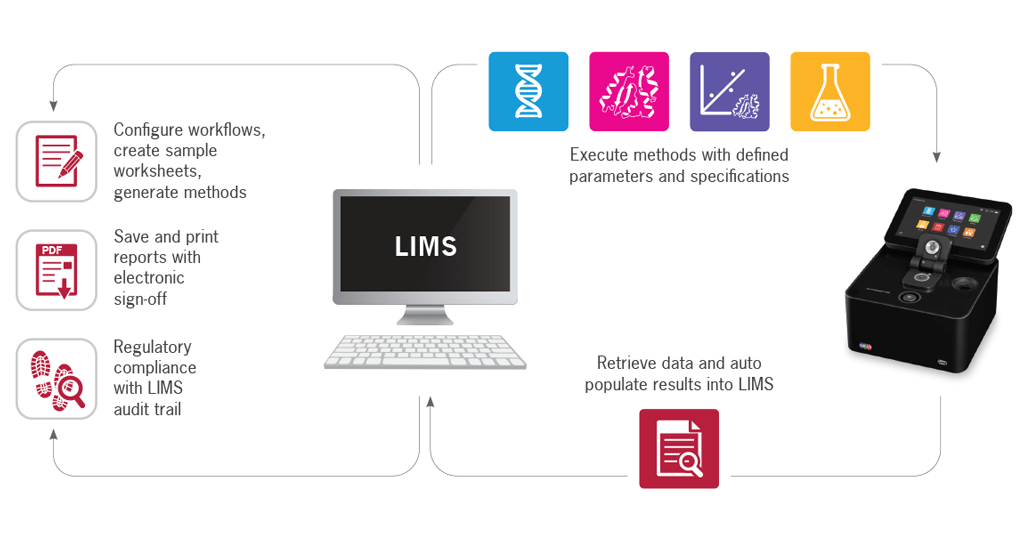
The first step in integration is establishing a communication link between the NanoPhotometer® and the LIMS. This often involves connecting the instrument to the laboratory network or directly to a computer where the LIMS is installed.

Data Transfer
Data Transfer
Integration enables the automatic transfer of data from the NanoPhotometer® to the LIMS. This includes measurement results, sample information, and any relevant metadata.
The NanoPhotometer® offers 3 levels of integration:
- Automatic upload of pdf and/or Excel files to a server drive within the LIMS system
- Transfer of specific data produced by the NanoPhotometer® either to a form or a template within the LIMS system the proprietary NanoPhotometer® REST API PUSH Service. Data are being acquired on the instrument using either the onboard software or computer app. The result data are automatically pushed to LIMS after each reading
- Execution of a method directly from LIMS and an automatic population of the measurement template with the obtained results coming from the instrument

Sample Identification
Sample Identification
The LIMS can provide sample IDs and associated information to the NanoPhotometer®. When a sample is loaded into the NanoPhotometer®, it can automatically associate the measurements with the correct sample ID, reducing the risk of data mix-ups utilizing the NanoPhotometer® IP address within the network.

Data Validation & Quality Control
Data Validation and Quality Control
The LIMS can implement data validation rules and quality control checks to ensure that the measurements from the NanoPhotometer® meet specific criteria before being accepted and recorded in the LIMS.
Data Storage
Data Storage
All data generated by the NanoPhotometer®, including absorbance readings and concentration calculations, will be securely stored within the LIMS. This ensures data integrity, accessibility, and long-term archiving.

Data Traceability
Data Traceability
Integration ensures that every data point generated by the NanoPhotometer® is traceable back to the instrument, the operator, the date and time of the measurement, and the specific sample or assay being analyzed. This traceability is crucial for regulatory compliance and auditing purposes.

Reporting
Reporting
The LIMS can generate customized reports based on the data collected from the NanoPhotometer®. These reports can be in various formats, including PDF, Excel, or custom templates, and can be easily shared with relevant stakeholders. In addition, the NanoPhotometer® also provides the option to produce pdf and Excel reports directly from the instrument.

Automation and Workflows
Automation and Workflows
Integration allows for the automation of laboratory workflows. For example, the LIMS can trigger additional assays or experiments based on the results obtained from the NanoPhotometer®.

User Authentication & Security
User Authentication and Security
Proper user authentication and security measures should be in place to control access to both the NanoPhotometer® and the LIMS, ensuring that only authorized personnel can operate the instrument or access the data.

Maintenance & Support
Maintenance and Support
Regular maintenance and support for the integrated system are essential to ensure its continued functionality and compliance with changing requirements and regulations. Even though the NanoPhotometer® instruments do not require maintenance or recalibration for a lifetime, it is good practice to run an IQOQ procedure on a regular basis. Implen is offering IQOQ either as a stand-alone option or as part of the CFR21 software package.